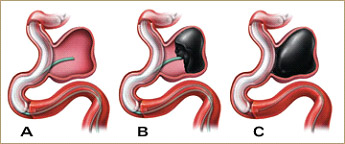
In late 2007 Onyx liquid embolic material became the first liquid embolic agent to be available in the United States for the treatment of intracranial aneurysms. Onyx HD-500 is an artificial, glue-like substance material used to block blood flow into aneurysms. The material is used to fill the aneurysm space, or pocket, and prevent the aneurysm from rupturing or increasing in size. Onyx 500 is FDA approved for the treatment of intracranial, saccular side wall aneurysms that present with a wide neck greater than or equal to 4 mm or with a dome to neck ration less than 2 that are not candidates for other techniques or in whom previous treatment has failed.
This material is an ethylene vinyl alcohol copolymer dissolved in the organic solvent dimethyl sulfoxide (DMSO) opacified with tantalum powder. Once coming into contact with an ionic solution the DMSO dissipates and the Onyx solidifies into a spongy, cohesive material.
The Onyx HD procedure is performed in a way very similar to the coil embolization procedure. However, instead of placing coils in the aneurysm, the liquid Onyx HD is used. The Onyx HD is carefully injected directly into the aneurysm through a small, thin micro-catheter while the bottom or base of the aneurysm is temporarily sealed with a separate balloon-tipped catheter.
The entire procedure takes about three hours, and patients usually stay in the hospital one or two days. Recovery time is very short, and many people return to work within in a week or two.